*(We don’t sell this item in Vietnam)
FacilityPro® Cleanroom Software by Particle Measuring Systems provides the interface, data management, and reporting for an environmental monitoring system. FacilityPro’s ability to manage viable, nonviable, and environmental data through a common system improves the efficiency of production operations and quality investigations.
With the FacilityPro industrial automation architecture, the data processing is performed by the Processor while the data management, display, and storage are performed by the software. This design approach increases system reliability and data integrity.
The software platform offers a number of intelligent features including data mapping, alarming, reporting, and recipe-driven sampling. Both also offer client software for remote access and viewing of data.
SCADA NG is built on the GE® Proficy® iFIX® SCADA NG platform and Historian database and provides several additional advanced features.
Features
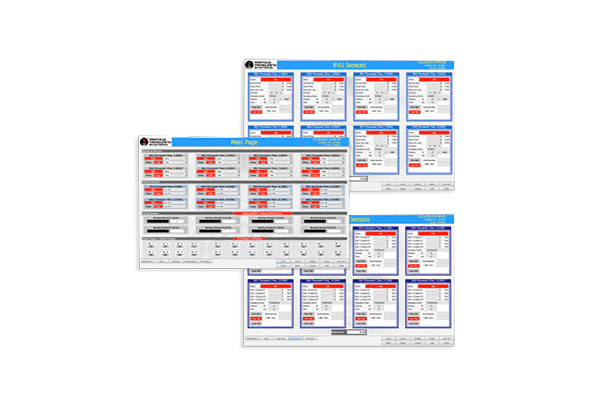
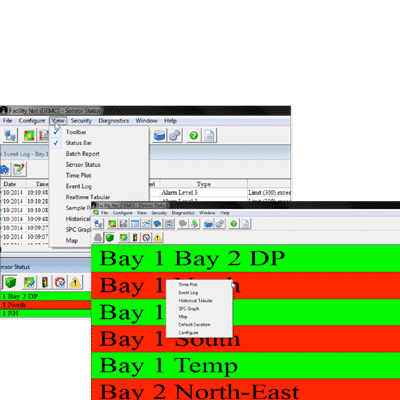
- Map for visual indication of viable, nonviable, and cleanroom environmental status
- Configurable display
- Alarm notification and e-signature acknowledgement
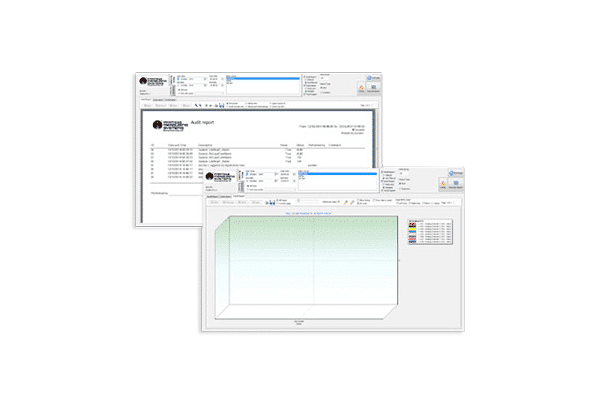
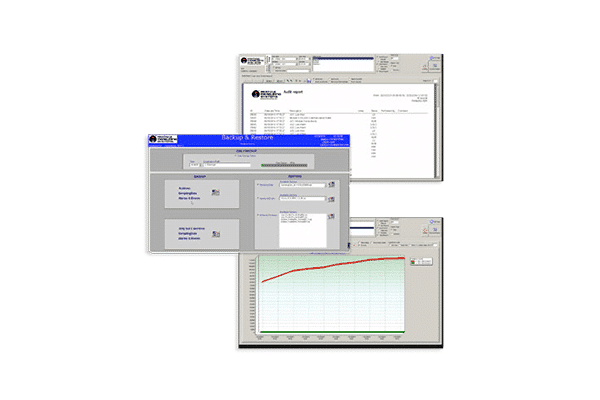
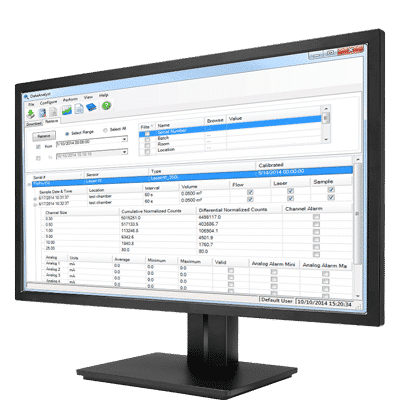
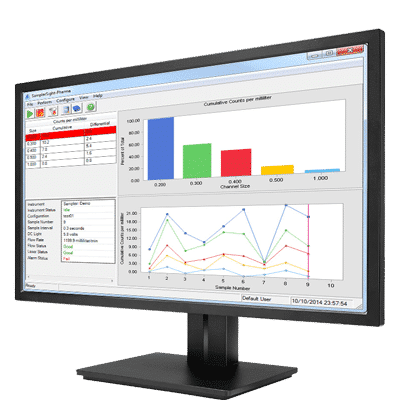
- Configurable reports including audit, statistics, and trend reports
- Batch identifiers and data filters
- Automation of sample collection, driven by recipes
- Cubic-meter algorithms for nonviable particle counting data
- Volume-based or time-based viable sampling
- Remote data viewing and reporting
Benefits
- Designed specifically for pharmaceutical cleanroom monitoring
- Integration and control of viable and non-viable sampling
- High data integrity
- Ultimate efficiency and reduced error
- Simple installation and validation
- Flexible integration
Applications
Monitoring of critical pharmaceutical environments including:
- Filling lines
- Isolators and RABS
- Blow-Fill-Seal
- Lyophilizer processes and transfer carts
- Biosafety cabinets and flow hoods
- General cleanroom and facility monitoring
Supporting Materials
Specification Sheets
FacilityPro® SMART NG / Pharmaceutical Net Pro Software >
FacilityPro® SCADA NG >
Pharmabilites Brochure >
Application Notes & Brochures
Paper Series: Guides to Particle Technology >
Planning and Installing a Facility Monitoring System >
21 CFR Part 11 and Data Integrity FAQ >
Data Integrity: Understand and Comply with GMP and FDA Requirements for 21 CFR Part 11 >
Assuring Data Integrity in an Environmental Monitoring System [Frequently Asked Questions] >
Particle Counter Data Collection and Interpretation for Pharmaceutical Manufacturing >
Webinars
Pharmaceutical Data Management for Root Cause Identification in Critical Environments (Case Study) >
Tackling the 21CFR11 Challenge: From Paper to Paperless >
Steps to Ensuring a Successful Audit – Effective Risk Assessment Design Webinar >
Private: EU GMP Annex 1 Review, Insights, and Feedback >
Understanding ISO Standards: 14644-2:2015 Cleanroom Monitoring (English) >