Sensitivity range: 0.3 – 25.0 µm; 1.0 CFM (28.3 LPM) – Built-in vacuum, VHP Resistant, POE
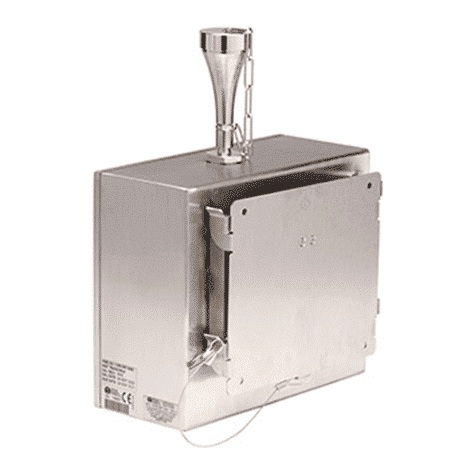
A compact, VHP resistant remote particle counter.
As part of a facility monitoring solution, the IsoAir® Pro-E Remote Particle Counter from Particle Measuring Systems (PMS) is an effective piece of a Contamination Control Strategy using the latest technologies to streamline cleanroom monitoring while meeting global regulations including EU GMP Annex 1, ISO 14644-2, and part of a 21CFR Part 11 solution. This remote particle counter has all the features you need for reliable particle monitoring in your clean area built into a simple but powerful package.
This is the first particle counter with a built-in vacuum designed for use with the Vaporized Hydrogen Peroxide (VHP) process. Additionally, this cleanroom monitoring instrument is powered by Power over Ethernet (POE) and with the internal blower, it requires very few external connections and supporting equipment.
Data from the instrument can be incorporated into either Particle Measuring Systems’ FacilityPro Software or Facility Net Monitoring System, with the option of third-party communications via Modbus or PMS protocol.
The quick-release mounting bracket stores essential sensor data such as the IP address at the point of measurement, reducing the time and complexity of unit installation after calibration or servicing. This remote particle sensor is “plug-n-play” with no complex re-programming before reinstallation into the particle monitoring system.
Highly user-friendly, the IsoAir Pro-E Remote Particle Counter has its own blower, eliminating the need for an external vacuum, and uses Power over Ethernet (PoE) to combine communications and power into a single connection. Additionally, the robust 316L stainless steel enclosure is liquid resistant with IP65 and NEMA 4X ratings which protect the unit during cleaning and disinfection activities.
The Isokinetic Sampling Probe (ISP) can be mounted directly to the inlet, extended from the particle sensor with rigid stainless-steel tubing, or remotely positioned with flexible tubing in virtually any installation.
Additionally, the IsoAir Pro-E is part of the PRO Series from PMS, a complete collection of contamination monitoring tools developed from a decades-long commitment to supporting pharmaceutical manufacturers. Use the IsoAir Pro-E together with other products in the PRO Series for a total contamination control solution and the assurance of up-to-date compliance. The PRO Series helps you provide lifesaving and life-changing solutions for your customers by supporting clean manufacturing.
Read more about the IsoAir Pro-E benefits and features below and explore what it can do for you today!
Product Details
Resources
Documentation
- SPEC SHEET: IsoAir Pro-E
- CATALOG: IsoAir Pro-Plus and Pro-E Spare Parts and Accessories
- OPERATIONS MANUAL: IsoAir® Pro-E Aerosol Particle Sensor
Educational Materials
- ISO 14644-2:2015 Cleanroom Monitoring
- Selecting The Most Suitable Particle Sample Point Locations In Your Cleanroom
- Review of Annex 1 2022
- ISO 14644-1:2015 Revisions Summary for Electronics
- Understanding ISO 21501-4 for Life Sciences Industries
Not sure where to start? Here are the next steps:

Tell us about your application requirements.

Our experts will help you find the right solutions to meet your specific requirements.

Once we identify the best solution for you, we provide you with pricing and delivery dates.