Replacing Settle Plates with Active Air Sampling
Settle plates were adequate in the past to meet regulations, but the new approach requires active air sampling.
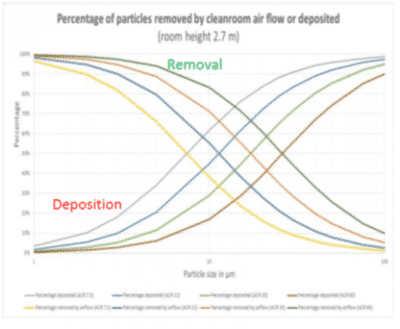
The most universally applied method for cleanroom classification is the one suggested in 1963 by Federal Standard 209 of the USA. In this standard the number of particles equal to and greater than 0.5 µm is measured in one cubic foot of air. This count is then used to classify the room (1 particle = class 1; 10 particles = class 10…). In the same period, scientific publications concerning healthcare facilities suggested that airborne particles carrying micro-organisms associated with human disease were usually found in the 4-20 µm equivalent diameter range. This contamination can be detected by settle plates.
Historically, cGMP guidelines highlighted their expectations concerning a microbiological continuous process monitoring in A and B grades (FDA guideline_2004 and Annex 1_2008) by referring to settle plates because no other technology was easily available. Unfortunately, settle plates are a non-validatable method as it is simply based on the physical principle of the downfall of a particle on a surface. We will discusses the using active air sampling versus passive air settle plate monitoring in routine environmental monitoring.
Complete this form to access this on-demand webinar
Ready to upgrade your contamination control strategy to include active air sampling? Particle Measuring Systems (PMS) has complete cleanroom contamination control solutions for you including:
- Our Contamination Control Advisory Services who can conduct a Risk Assessment for your pharmaceutical processes and provide a variety of support.
- Active air samplers including:
- portable/mobile
- fixed/remote
- single use
- Data management software from Particle Measuring Systems
Particle Measuring Systems is direct in every major market and able to ensure the same ongoing support no matter where you are located. Contact us today for a quote.