Case Study: Developing an Isolator Filling Line Using Quality Risk Management in Pharmaceuticals
Following the patent of the “Espresso” innovative infusion device, the Italian Biochemical Institute (IBI) “Lorenzini” commissioned Comecer to construct a new isolated filling line. Particle Measuring System’s Contamination Control Advisory Team oversaw the development of the aseptic filling process and the environmental monitoring system of this project by applying proven quality risk management techniques. Using this case study we will provide practical insights for Quality by Design principles.
Key Learning Points
- Project Management Theory
- Project milestone examples
- Analyzing, reviewing, and improving processes
- Process steps risk analysis and mitigation
- Defining air and surface critical sampling points
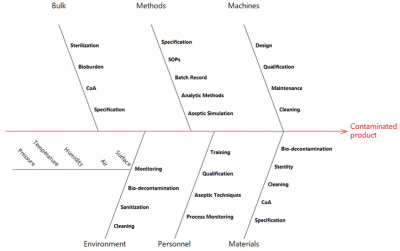
- Using risk management tools (FMEA, Fishbone, HACCP)
- FAT/SAT and validation approach
COMPLETE THE FORM TO ACCESS THIS ON-DEMAND WEBINAR
Particle Measuring Systems (PMS) has complete contamination control solutions for you including:
- Our Contamination Control Advisory Services who can conduct a Risk Assessment for your pharmaceutical processes
- Cleanroom particle counters including portable/mobile and fixed/remote
- Microbial Monitors including portable/mobile, fixed/remote, and single use.
- Data management software
We are direct in every major market and able to ensure the same ongoing support no matter where you are located. Request a quote now.