The revised ISO 14644-1:2015 has set new cleanroom classification and monitoring standards for cleanroom managers and manufacturers, causing some questions and confusion.
ISO 14644 expert, Daniele Pandolfi (of Particle Measuring Systems) has written a brief but comprehensive paper that includes:
- a summary of the new cleanroom classification standards, highlighting the changes
- an explanation of how it affects your cleanroom monitoring
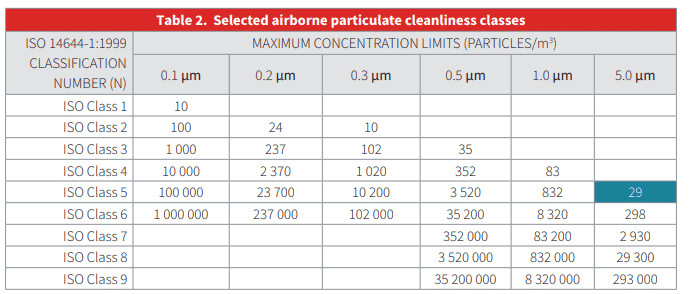
- ISO 14644-1 cleanroom classification table
- Cleanroom counts per ISO 14644-1
- Cleanroom particle count limits
This paper highlights the major changes in the new ISO 14644-1 compared to the previous version, as well as the possible impact on the Pharmaceutical EU GMP Annex 1 and FDA Aseptic Processing Guideline and includes information on ISO 14644-2 and ISO 21501-4.

Interested in learning more?
Complete the form to get the full paper.
Learn about the latest Annex 1 approach for Cleanroom classification and monitoring.
Read about ISO 14644-2 Cleanroom monitoring requirements.
Particle Measuring Systems (PMS) has complete cleanroom classification and monitoring solutions for you including:
- Our Contamination Control Advisory Services who can conduct a Risk Assessment for your pharmaceutical processes
- PMS Cleanroom particle counters including portable/mobile and fixed/remote
- Microbial Monitors including portable/mobile, fixed/remote, and single use.
- Data management software from Particle Measuring Systems
Particle Measuring Systems is direct in every major market and able to ensure the same ongoing support no matter where you are located.

AUTHOR
Sr. Daniele Pandolfi
Daniele Pandolfi é o Gerente Global de Linha de Produtos de Software/Sistemas/Serviços da Particle Monitoring Systems, Inc. Com mais de 11 anos de experiência em contador de partículas e controle de contaminação em salas limpas, ele se concentra em construir e manter relacionamentos fortes com os clientes. Ele ajudou muitos usuários de salas limpas a resolver seus problemas de cGMP.
