Continuous & Effective Microbiological Air Monitoring in Critical Environments
A Comparison of Microbial Analytical Methodologies
Manufacture of sterile products must strictly follow carefully established and validated analytical methods of manufacture and control. Based on this consideration, we evaluated scientific literature describing settle plates and active air sampler monitoring effectiveness. A contamination control strategy should be implemented by pharma manufacturers, especially for aseptic productions, to assess the effectiveness of environmental monitoring and demonstrate that the process is under statistical control. It is of key importance for microbiological monitoring data to correlate as best as possible with total particle monitoring data so that each batch release is reliably supported.
The full paper is published in the
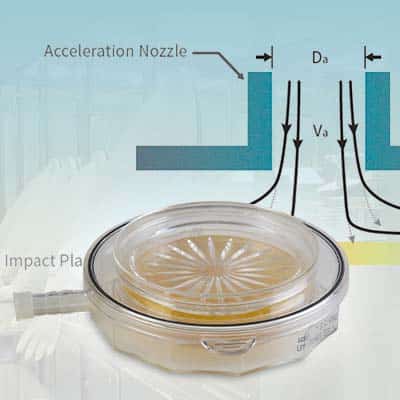

Looking for an effective continuous microbial air monitoring solution? Check out the BioCapt® Single-Use Microbial Impactor, the innovative replacement for settle plates. Full specifications and a testimonial from our experts are available by filling out the form on this page.
