Compressed Gas Microbial Monitoring in Aseptic Manufacturing
Microbial Survival in Compressed Gases Under Fast Decompression to Normal Atmospheric Conditions
Compressed gas microbial monitoring in aseptic manufacturing presents particular challenges. The FDA guideline for the manufacturing of sterile products using aseptic processes specifically mentions that a compressed gas should be of appropriate purity (e.g., free from oil) and its microbiological and particle quality after filtration should be equal to or better than that of the air in the environment into which the gas is introduced.
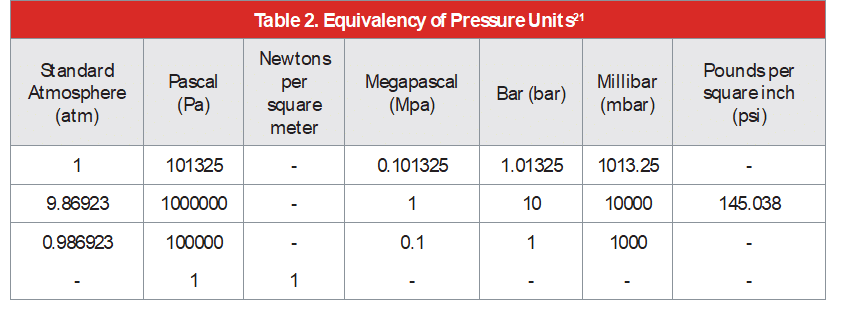
Additional guidance on cleanroom or clean air monitoring and limits of particulate and microbial contaminants in the pharmaceutical industry is provided in EU GMP Annex 1 20082 , and the USP chapter 11163 . A more specific reference is ISO 8573, a group of international standards relating to the quality (or purity) of compressed air. The standard consists of nine separate parts, with part 1 specifying the quality requirements of the compressed air4 and parts 2 – 9 specifying the methods of testing for a range of contaminants. The test method for microbial monitoring of compressed gases is provided in ISO 8573-75 .
Interested in Learning More?
Complete the form to download the complete paper.
Looking for a microbial monitoring solution? Particle Measuring Systems (PMS) has a wide range of portable and remote microbial viable samplers including:
- MiniCapt Active Air Samplers available in a variety of portable or remote models
- use with the compressed gas kit
- BioCapt Impactors available as a single use solution or stainless steel
