Effective Compressed Gas Environmental Monitoring: Particles and Microbials
Control of the environment in which pharmaceutical products are manufactured is a key element of Good Manufacturing Practices (GMP). Environmental monitoring of particles and microorganisms in manufacturing cleanrooms, Restricted-Access Barrier Systems (RABS), and isolators consists of clearly defined components.
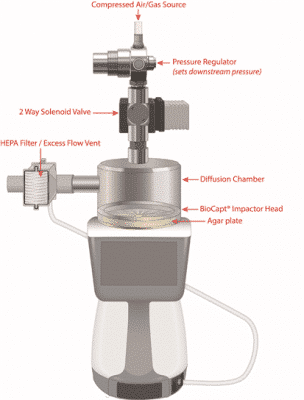
Absence of particle and microbial (VNV) contamination is considered a critical quality attribute due to its potential to dramatically impact, directly or indirectly, the safety and/or the efficacy of the drug product. Moreover, compressed gases can be viewed as critical utilities in pharmaceutical Industry when either in direct product contact or directly entered into the clean room environment.
This on-demand webinar covers regulatory guidelines as well as providing practical solutions for compressed gas environmental monitoring.
COMPLETE THE FORM TO WATCH THIS ON-DEMAND WEBINAR
Get the Questions and Answers from this webinar.
Ready to start monitoring your compressed gases? Particle Measuring Systems has a variety of solutions for both particle and microbial monitoring. Learn more here.