Paper: Understand & Comply with GMP & FDA Data Integrity Requirements
This data integrity paper, “Understanding and Becoming Compliant with GMP and FDA Requirements”, discusses data (such as environmental monitoring data or data from personnel) requirements and management. After reading this paper you will better understand 21 CFR Part 11 data requirements including data management and electronic signatures.
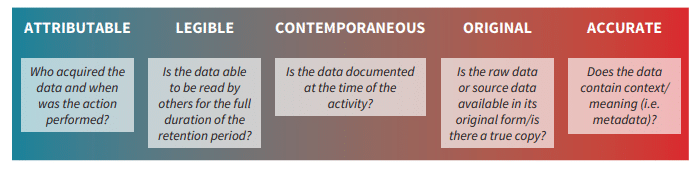
Data integrity means data (such as from personnel or environmental monitoring) that is accurate, complete and repeatable, which in turn ensures the product’s quality and public safety. In recent years, infractions relating to data integrity have been noted in several Food and Drug Administration (FDA) warning letters, but it is not a new concept. The importance of record-keeping in drug manufacturing can be seen as far back as 1938, when the Federal Food, Drug, and Cosmetic (FDC) Act required the safety of new drugs be documented before being sold to the public, with similar regulations instigated in Europe and Japan throughout the 20th century.
.
Production systems have large, inherent operational risks and are difficult to validate. Instead of only being reactive to public health disasters, preventative measures, such as the requirement for proof of claims, are taken to lower their likelihood and propagate confidence in manufacturers.
Read this paper to learn about 21 CFR Part 11 data integrity requirements. The document is divided into three parts:
- General Provisions
- Electronic Records
- Electronic Signatures
Complete the form to get the full paper.
