A laboratory information management system (LIMS), also known as a laboratory information system (LIS) or laboratory management system (LMS), is a software-based solution with features that support a modern laboratory’s operations. LIMS is dedicated to helping pharmaceutical, biotechnology, and other life sciences organizations leverage their investment in information technology and quality processes. In order to address pharmaceutical and biotechnology companies’ most pressing issues including:
• Speeding up time to market
• Reducing operating & product costs
• Achieving high-quality standards
• Providing product governance & compliance with regulatory agencies
What is LIMS Laboratory Information Management System?
LIMS is a powerful and scalable laboratory information management system designed to meet the production and clinical needs of all modern laboratories. It is a business-ready solution that automates all laboratory information, from experiments to manufacturing offering a complete enterprise solution that is easy to deploy, maintain, upgrade, and integrate with other organizational business systems.
LIMS has allowed laboratories to cut back manual and time-consuming activities and has empowered pharmaceutical and biotechnology organizations to achieve decreasing product time to market while ensuring regulatory compliance. Over the years, the importance of system integration and automation has drastically increased, thus using LIMS at the forefront of process harmonization and data centralization. LIMS offers the entire organization with seamless integration between laboratory testing, scheduling, data collection, and real-time reporting.
With thousands of customers, LIMS is one of the most widely used and trusted applications in the life science industry today. LIMS is not only a business-ready modular system but as well as an intuitive integrated application that allows quality departments to make decisions based on all the available information and address an organization’s immediate quality requests and needs.
What are the key advantages of LIMS?
• Improved efficiency
• Increased visibility
• Decreased cycle time
• Reduced risk
• Improved decision-making
• Increased accountability
• Rapid ROI
• Data Integrity
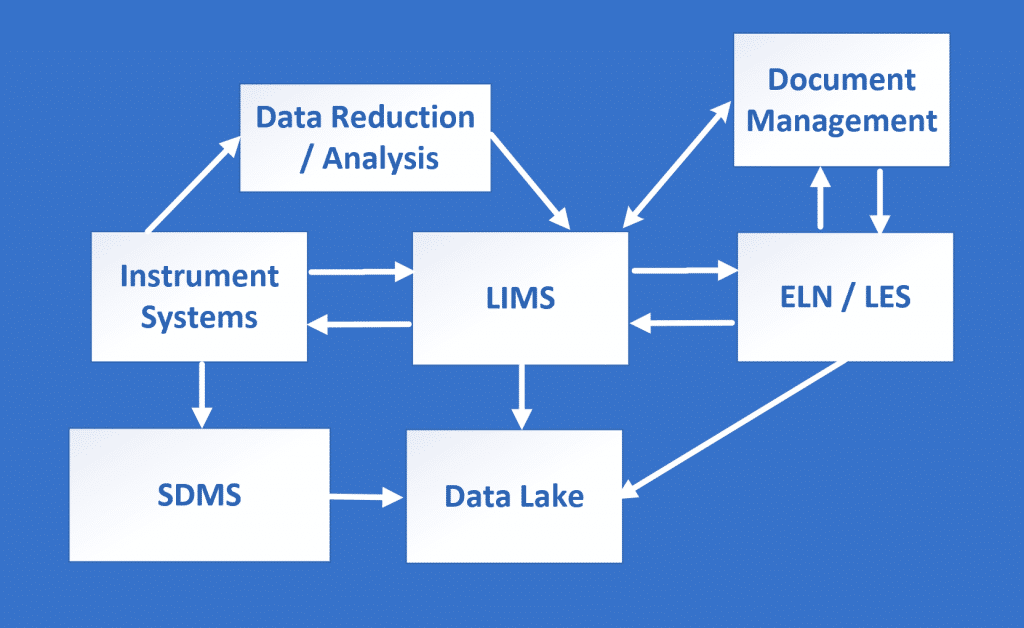
Do you need improved laboratory informatics systems?
LIMS, ELN, LES, SDMS, and CDS can contribute significantly to increasing efficiency in the lab, releasing time for core science activities, and enabling a wider audience to utilize valuable scientific data.
These systems come in a variety of formats suited for different industries and processes. It is essential for Chromatographic Analysis.
Some of the typical laboratory systems
CDS (Chromatography Data Management System) (Hệ thống dữ liệu sắc ký)
A chromatography data system (CDS) is a set of dedicated data-collection tools that integrate with a laboratory’s chromatography equipment. CDS plays a pivotal role in instrument control, data processing, report generation, and data archiving. It is essential for Chromatographic Analysis, including high-performance liquid chromatography (HPLC), ion chromatography (IC), gas chromatography (GC),…which constitutes a major portion of testing performed in analytical laboratories.
CDS is beneficial because it automates the collection of data, allowing one to perform tasks without having to manually manage datasets. Therefore, reducing user error and ensuring that the information. It also ensures that laboratory workflows and practices meet the strict regulatory standards that surround data integrity.
ELN (Electronic Lab Notebook) (Sổ ghi chép điện tử)
Electronic Lab Notebook is a software tool that in its most basic form replicates an interface much like a page in a paper lab notebook.
LES (Laboratory Execution Systems) (Hệ thống thực hiện phòng thí nghiệm)
A laboratory execution system is a laboratory informatics solution employed in the laboratory, at the analyst work level, to aid in step enforcement for procedural laboratory test method execution.
LIS (Laboratory Information System) (Hệ thống thông tin phòng thí nghiệm)
A laboratory information system is a software solution that processes, stores, and manages data related to laboratory processes and testing. In order to coordinate the workflow and quality control of testing, and database as well for future reference
SDMS (Scientific Data Management System) (Hệ thống quản lý dữ liệu khoa học)
A scientific data management system (SDMS) acts as a centralized document management system, collecting, cataloging, and storing data generated by and in a scientific lab. An SDMS is designed to handle unstructured data from data systems like a LIMS or a ELN.