Get the latest Annex 1 draft insights on definitions and guidance regarding Cleanroom Classification, Qualification, and Monitoring.
In this paper, Maurizio Della Pietra, MPhys, of Particle Measuring Systems (PMS), discusses various aspects of Cleanroom Classification, qualification and monitoring including:
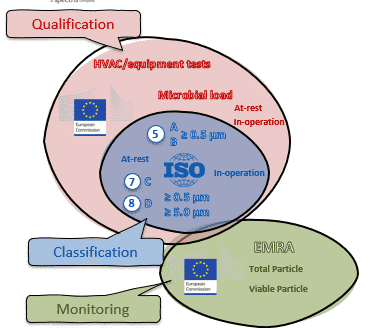
- When a Risk Assessment is important
- Which particle sizes to measure
- Definitions such as cleanroom classification vs. cleanroom qualification
- Requirements for re-qualification
- Cleanroom monitoring and it’s relationship to cleanroom classification and qualification
- Cleanroom monitoring verification
- Solutions to meet the new Annex 1 expectations
Interested in learning more on cleanroom classification, qualification, and monitoring?
Complete the form to get the full paper.

Particle Measuring Systems (PMS) has complete cleanroom contamination control solutions for you including:
- Our Contamination Control Advisory Services who can conduct a Risk Assessment for your pharmaceutical processes
- PMS Cleanroom particle counters including portable/mobile and fixed/remote
- Microbial Monitors including portable/mobile, fixed/remote, and single use.
- Data management software from Particle Measuring Systems
Particle Measuring Systems is direct in every major market and able to ensure the same ongoing support no matter where you are located.
